
Anemia
Iron deficiency anemia was associated with 39% increased odds of stroke, independent of the risk factors for stroke.
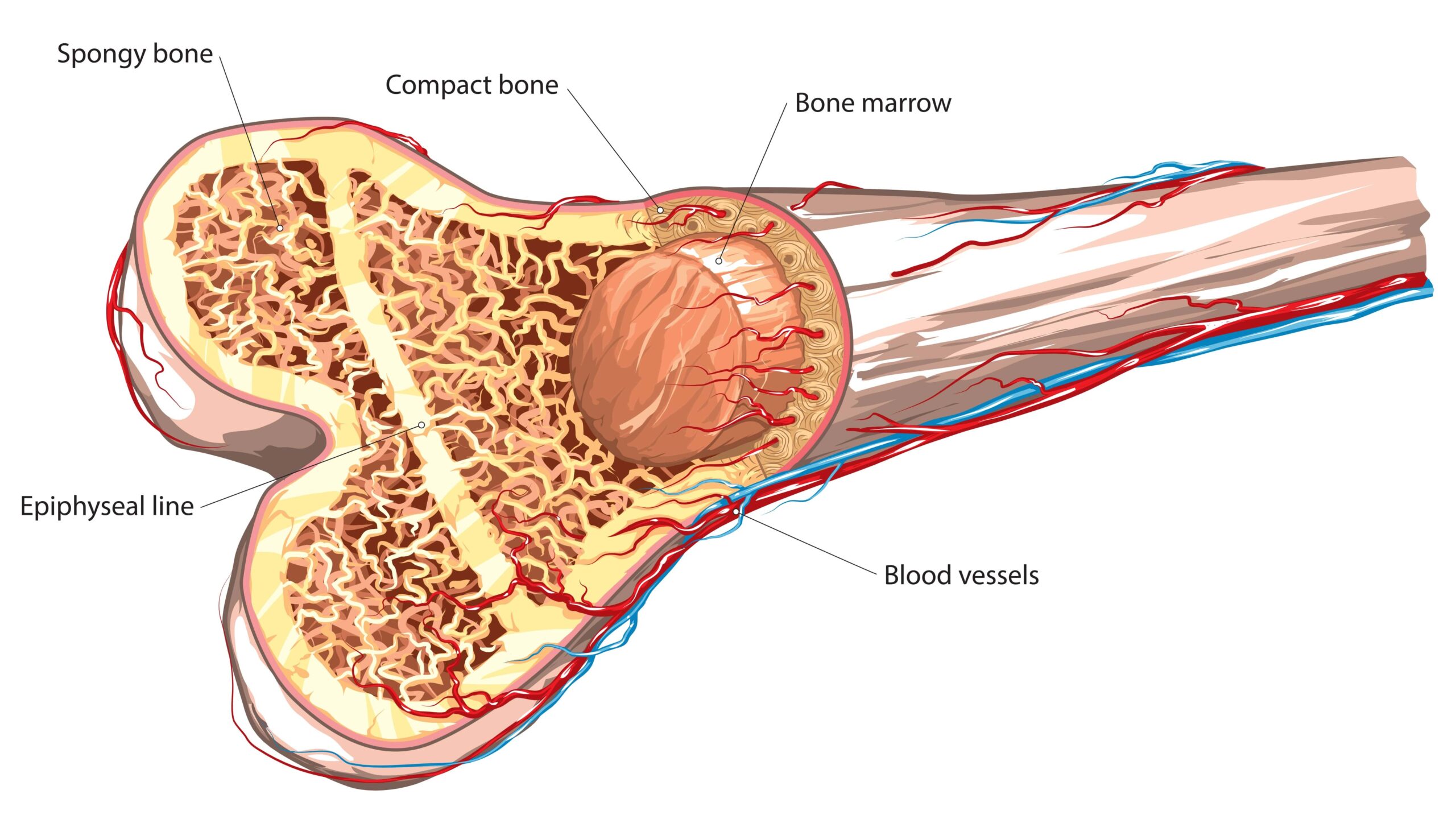
Reactive oxygen species levels decreased and patients with evident bone marrow failure showed hematological response.
The study led by Paul George, MD, PhD, also highlights the importance of patient regimen adherence to secure these benefits.
In a phase 1 trial the product performed favorably in aplastic anemia, myelofibrosis, and hypoplastic myelodysplasia.
The FDA granted orphan drug designation to rilzabrutinib for warm autoimmune hemolytic anemia and IgG4-related disease.
The oral agent exhibited promising hemoglobin response performance and safety in this stage of an ongoing phase 2/3 study.
Imetelstat is indicated for patients with lower-risk MDS who had an unsatisfactory response to or are ineligible to ESAs.
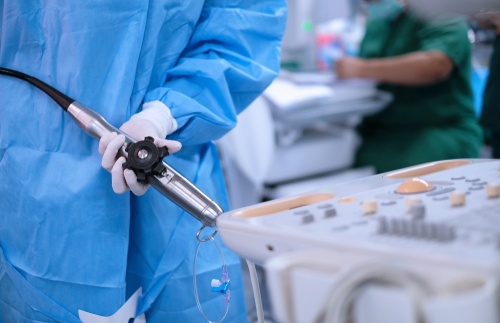
A prospective study involved ultrasound imaging of patients' carotid arteries to evaluate for structural changes.
Advertisement
Expert Interviews on Hematology
Knowledge Hubs
Advertisement





 © 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.
© 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.