
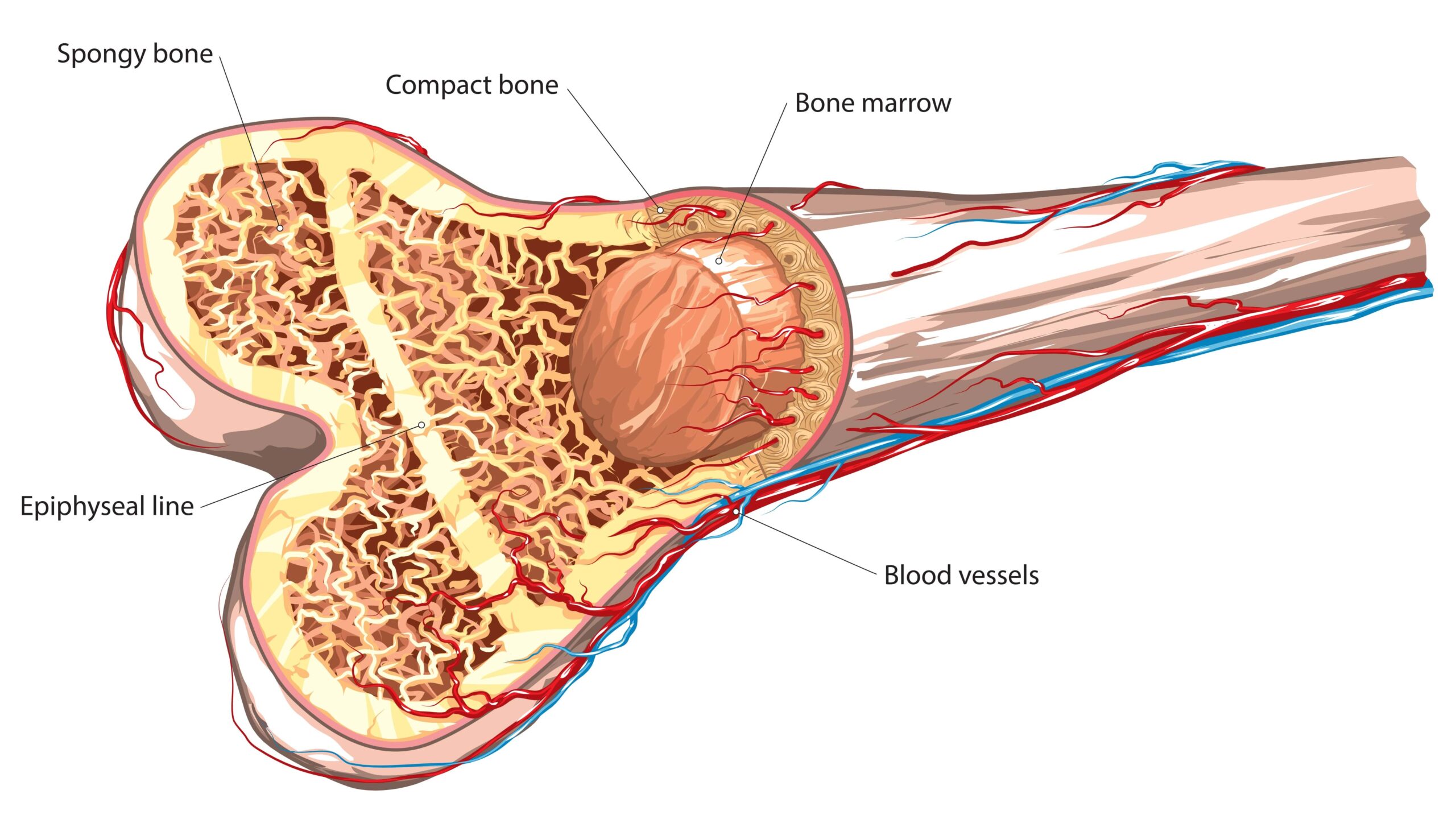
Myeloproliferative Neoplasms
The SURPASS ET trial evaluated efficacy and safety of ropeginterferon alfa-2b in second-line ET treatment.
The study data presented at EHA2025 were from patients who had disease resistant to prior therapy or intolerance to therapy.
The FDA previously granted Orphan Drug Designation to nuvisertib for use in myelofibrosis in May 2022.
MANIFEST-2 compares pelabresib plus ruxolitinib with placebo plus ruxolitinib for JAK-inhibitor-naive disease.
While cytoreductive drugs did not reduce thrombosis risk, interferon significantly improved myelofibrosis-free survival.
Reactive oxygen species levels decreased and patients with evident bone marrow failure showed hematological response.
The agent targets abnormal gene expression in order to control the excessive cell proliferation that occurs in PV.
Adrián Mosquera Orgueira, MD, PhD, spoke of his team's study into machine learning tools for improving risk stratification.
In a phase 1 trial the product performed favorably in aplastic anemia, myelofibrosis, and hypoplastic myelodysplasia.
Investigational T-Cell Immunotherapy Superior to Standard HSCT in Rate of Survival Plus GvHD Freedom
A phase 3 study comparing the treatments in multiple hematologic malignancies also saw greater overall survival at one year.Advertisement
Expert Interviews on Hematology
Knowledge Hubs
Advertisement






 © 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.
© 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.