Sleep tourism vacations are designed to optimize rest, and are on the rise, reflecting a growing societal shift toward prioritizing wellness and sleep health. In this interview, Dr. Anne Marie Morse emphasizes that while sleep tourism offers curated environments with sleep-friendly features and temporary relief from daily stressors, it should not be mistaken for a medical solution to chronic sleep issues. She advocates for a more integrated approach where resorts partner with sleep and circadian health experts to create sustainable strategies that extend beyond the vacation.
Advertisement
ChatGPT shows limited accuracy and consistency in breast cancer care, highlighting the need for expert clinical judgment.
In a cohort of adults and adolescents marstacimab produced bleed control superior to that from on-demand bypassing agent.
Research revealed an association between social isolation and the onset of CVD and accelerated death in CKD.
Ensifentrine offers a novel, inhaled option to improve COPD symptoms in patients not controlled on dual bronchodilators.
Targeting fibroblast ACSL4 may offer a more effective, precise therapy for IBD by blocking ferroptosis in epithelial cells.
Genetic targets of iron supplements may raise IBD risk—highlighting need for caution in treatment strategies.
New study finds medicinal leeches unlikely to spread hepatitis B—even after feeding on infected blood.
A study found that primary care physician-nephrologist collaboration did not improve kidney or CV outcomes in stage 5 CKD.
DocWire Content Partners
Powered by DocWire News
The Latest From GI Oncology Now
Suvemcitug, envafolimab, and FOLFIRI may serve as a new second-line treatment option for cold tumors.
KRASG12C inhibitors can have reduced efficacy in patients with alterations in KRAS, EGFR, and other genes.
Complete responders with HCC have prolonged survival and durable disease control even after therapy has been discontinued.
The primary objective of median PFS was significantly longer for patients treated with 177Lu-edotreotide.
The safety profile of the combination was consistent with known profiles, and no new safety findings were observed.
Older patients in their 70s with extrahepatic CCA have a higher rate of choledocholithiasis.
The Latest From Heme Today
In a cohort of adults and adolescents marstacimab produced bleed control superior to that from on-demand bypassing agent.
The agent notably in preclinical studies has accomplished increased HbF without any evident cytotoxicity.
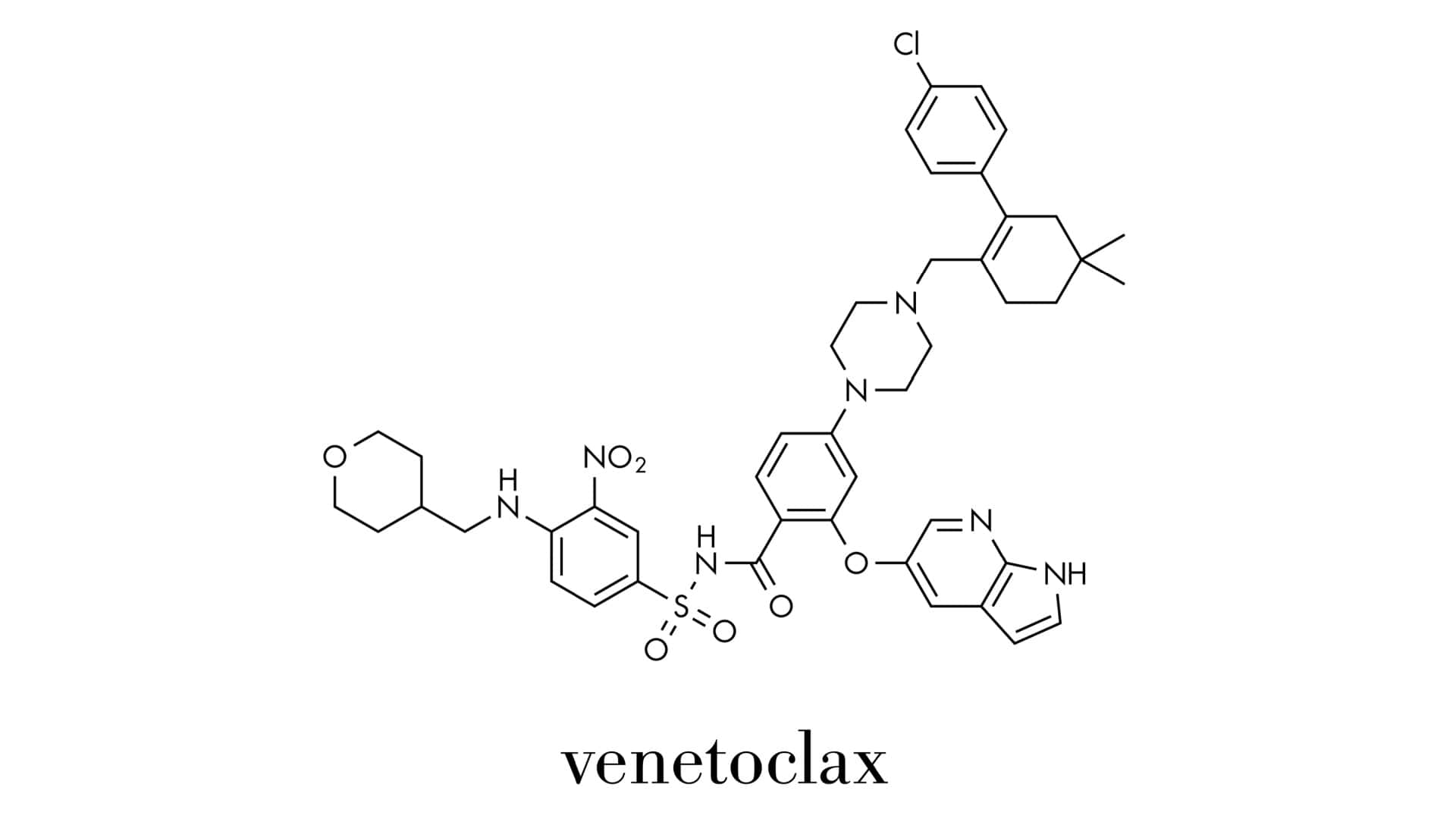
The phase 3 VERONA trial, which compared this combination with placebo plus azacitidine, observed no new safety signals.
Dr. John Gansner and Dr. Martina H. Slingsby argue this new agent can provide benefit across all bleeding disorders.
Heme Today spoke with Ulrike Reiss, MD, and Andrew Davidoff, MD, of the trial's investigator team.
Gene therapy is reshaping sickle cell care, raising awareness and advancing treatments beyond the lab.































 © 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.
© 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.