Heme
Advertisement
Evidence was presented at EHA2025 that the agent improves RBC health and blood flow in patients.
Two cases of patients with SCD and history of delayed hemolytic transfusion reaction were presented at EHA2025.
The analysis was an assessment of 15-year follow-up data from the CLIMB study and was presented at EHA2025.
The study data presented at EHA2025 were from patients who had disease resistant to prior therapy or intolerance to therapy.
The FDA previously granted Orphan Drug Designation to nuvisertib for use in myelofibrosis in May 2022.
Vivien Sheehan, MD, explained how a novel assay can guide personalized therapeutic approaches for sickle cell disease.
Rilzabrutinib significantly decreased spleen weight and reduced inflammation in mice with homozygous sickle cell genotype.
MIRCA is a device designed to assess the flexibility of red blood cells of patients with sickle cell disease.
The study will assess the overall response to olutasidenib plus azacitidine in higher-risk MDS, CMML, and advanced MPNs.
In a study, patients with T2DM and MGUS who used GLP-1 agonists were observed over 10 years to have reduced MM rates.
Arm D of the SEQUOIA trial evaluated the combo in patients with either del(17p) or TP53 mutation, both mutations, or neither.
The results are reported from an international cohort and support adding the agent to plasma exchange and immunosuppression.
MANIFEST-2 compares pelabresib plus ruxolitinib with placebo plus ruxolitinib for JAK-inhibitor-naive disease.
In a phase 3 study, patients who received recombinant ADAMTS13 did not experience acute TTP events during prophylaxis.
Iron deficiency anemia was associated with 39% increased odds of stroke, independent of the risk factors for stroke.
While cytoreductive drugs did not reduce thrombosis risk, interferon significantly improved myelofibrosis-free survival.
A preclinical mouse study showed hemostasis restoration, as well as use for active bleeding and internal bleeding prevention.
A novel complement-targeting agent shows impressive efficacy and safety without immunosuppression of the classical pathway.
Investigation of a HUNT Study cohort found elevated plasma MBL levels to be associated with increased risk for future VTE.
Reactive oxygen species levels decreased and patients with evident bone marrow failure showed hematological response.
Advertisement







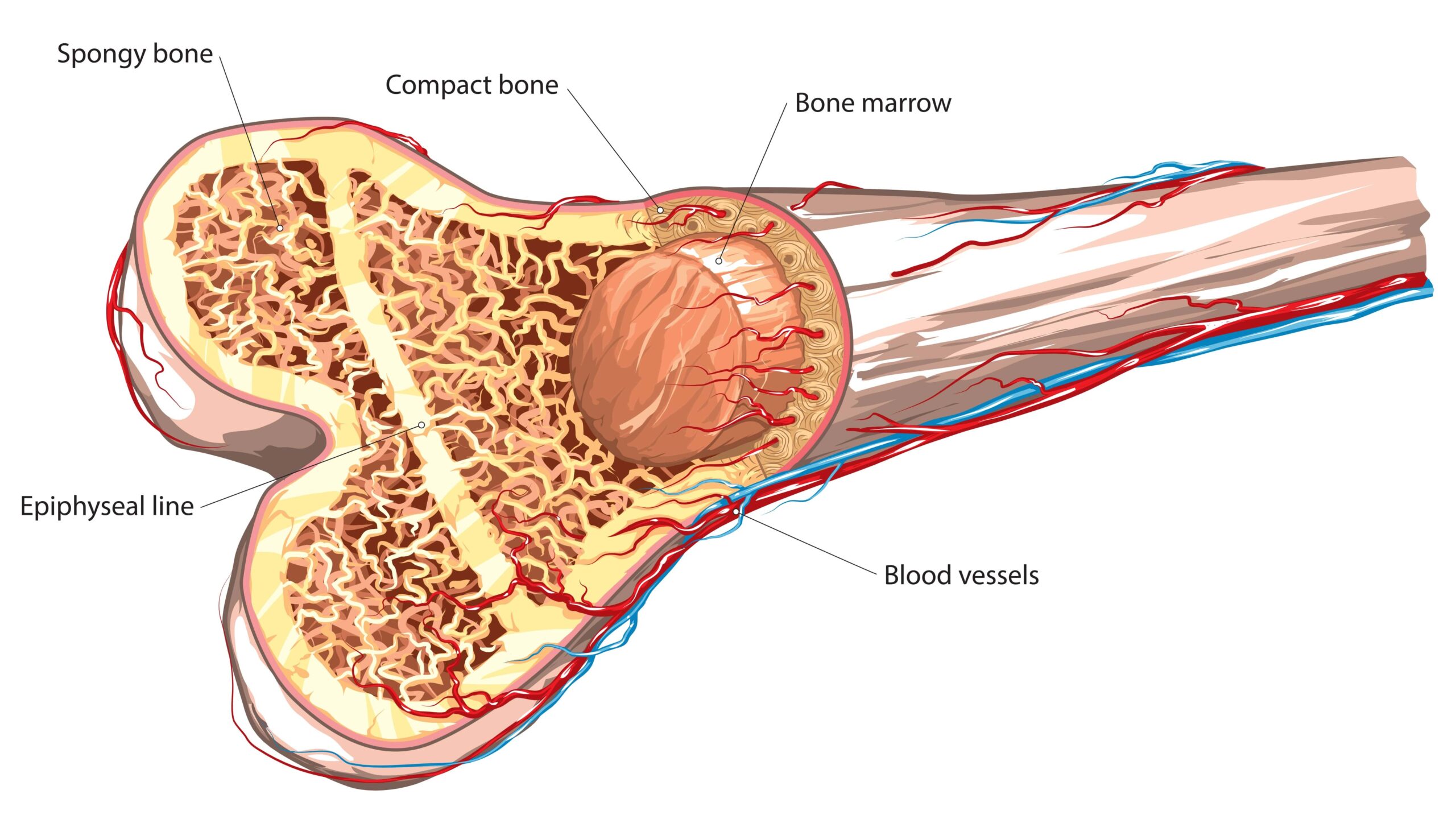

 © 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.
© 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.