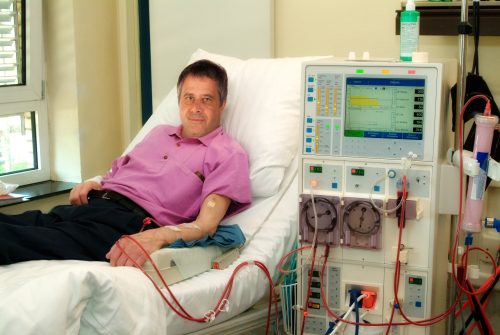
In a February 2023 press release, the FDA announced approval of the first oral agent for chronic kidney disease (CKD)-related anemia in adults on dialysis. Daprodustat is a once-daily tablet specifically indicated for patients who have been receiving dialysis for at least 4 months. Other FDA-approved treatments for CKD-related anemia are injected into the blood or under the skin.
Ann Farrell, MD, director of the division of nonmalignant hematology in the FDA Center for Drug Evaluation and Research, said, “With an oral drug in addition to the FDA-approved injection options, adults with chronic kidney disease on dialysis now have multiple ways to trat their anemia. This approval demonstrates the FDA’s commitment to helping bring a range of therapeutic options to patients with chronic diseases. Patients can consult with their health care providers to select the option that is most appropriate.”
Daprodustat increases erythropoietin levels. The drug’s effectiveness was demonstrated in a randomized study of 2964 adults receiving dialysis (NCT02879305). Adults in the study received either oral daprodustat or injected recombinant human erythropoietin (a standard of care for patients with CKD-related anemia). Daprodustat raised and maintained hemoglobin within the target range of 10-11 g/dL, similar to the efficacy of recombinant human erythropoietin.
The drug carries a boxed warning for an increased risk of blood clotting events including death, heart attack, stroke, and blood clots in the lungs, legs, or dialysis access site. Other warnings and precautions include a risk of hospitalization for heart failure, worsening increase of blood pressure, stomach erosions, and gastrointestinal bleeding. The most common side effects are high blood pressure, thrombotic vascular events, abdominal pain, dizziness, and allergic reactions.
Daprodustat is not approved for patients not on dialysis.





 © 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.
© 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.