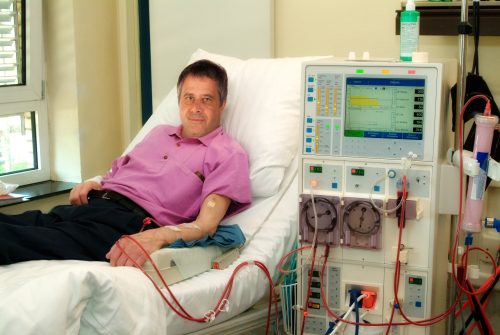
Patients undergoing maintenance dialysis for chronic kidney disease who do not have hyperparathyroidism will not reduce their risk of certain cardiovascular events with oral alfacalcidol, according to a new study.
The study randomized 976 patients from 108 dialysis centers in Japan receiving maintenance hemodialysis with serum intact parathyroid hormone levels less than or equal to 180 pg/mL between Aug. 18, 2008, and Jan. 26, 2011. Final follow-up was on April 4, 2015. Patients were treated either with 0.5 μg of oral alfacalcidol per day (intervention group; n = 495) or without vitamin D receptor activators (control group; n = 481). Primary outcomes were fatal and nonfatal cardiovascular (CV) events, myocardial infarctions, hospitalizations for congestive heart failure, stroke, aortic dissection/rupture, amputation of lower limb due to ischemia, and cardiac sudden death; coronary revascularization; and leg artery revascularization. Secondary outcome was all-cause mortality.
Oral alfacalcidol, a vitamin D receptor activator (VDRA), is not associated with reduced cardiovascular risk among patients without secondary hyperparathyroidism receiving maintenance hemodialysis. @JAMA_current
— PhysiciansFirstWatch (@Physns1stWatch) December 13, 2018
Of the total cohort, 944 patients completed the trial (intervention group, 488; control group, 476). CV events occurred in 21.1% (n = 103/488) of the intervention group and 17.9% (n = 85/476) of the control group (absolute difference, 3.25% [95% CI, −1.75% to 8.24%]; hazard ratio, 1.25 [95% CI, 0.94-1.67]; P = .13). All-cause mortality rates were 18.2% in the intervention group and 16.8% in the control group (hazard ratio, 1.12 [95% CI, 0.83-1.52]; P = .46). Serious CV-related adverse events occurred in 40.8% (n = 199) of the intervention group; 13.1% (n = 64) sustained an infection, and 4.5% (n = 22) experienced malignancy-related serious adverse events. In the control group. 40.1% (n = 191) had a serious CV-related adverse event, while 13.2% (n = 63) and 4.4% (n = 21) experienced serious infection and malignancy-related adverse events, respectively.
Alfacalcidol Does Not Lower CV Risk in Dialysis, Trial Suggests https://t.co/lp9cn4lrlu via @medscape
— Irish Medicines Formulary (IMF) and IMF-Online (@FormularyIE) December 17, 2018
Effect of Oral Alfacalcidol on Clinical Outcomes in Patients Without SecHPT Receiving Maintrnance Hemodialysis: The J-DAVID Randomized Clinical Trial. J-DAVID Investigators, Shoji T et al. JAMA. 2018 Dec 11 https://t.co/A0Q4ypgE2B …no significant effect pic.twitter.com/6ZkaIfzwBU
— Osteoporosis NL (@Osteoporosis_NL) December 13, 2018
The study’s findings appear in JAMA.
The researchers wrote, in sum, “Among patients without secondary hyperparathyroidism undergoing maintenance hemodialysis, oral alfacalcidol compared with usual care did not reduce the risk of a composite measure of select cardiovascular events. These findings do not support the use of vitamin D receptor activators for patients such as these.”
Open-label RCT of alfacalcidol vs. control shows no effect on CVD in ESKD. Some reservations re. trial design and inclusion criteria aside, I can’t say I’m surprised. Worry that much (if not) all we do to treat MBD in clinical practise is useless for CVD… https://t.co/wb8KAQ8zuo
— Iain Bressendorff, MD PhD (@IBressendorff) December 12, 2018
VITAL: Omega-3 and Vitamin D Supplements Did Not Reduce Major CVD Events, Invasive Cancers
Study: Vitamin D Supplements Do Not Affect Bone Health
Prevalence of vitamin D deficiency in postmenopausal high- and low-energy fracture patient
Source: JAMA






 © 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.
© 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.