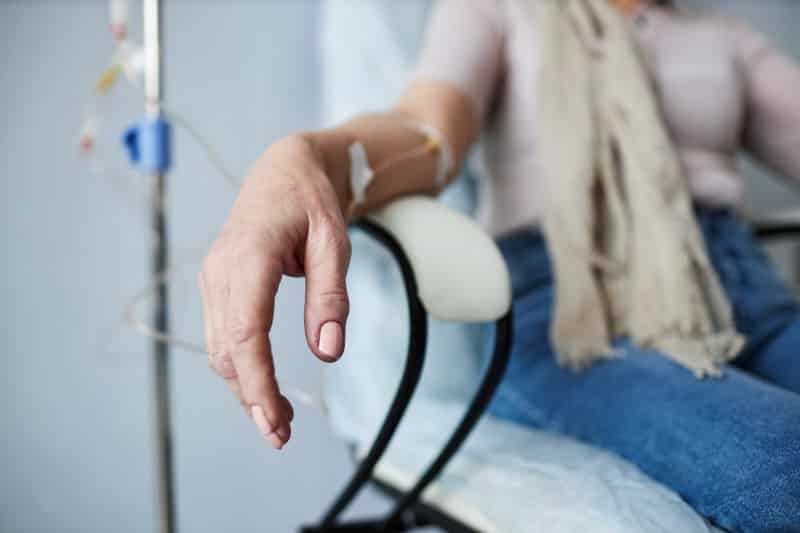
The US Food and Drug Administration (FDA) has granted 510(k) clearance to TabloCart with prefiltration, an optional accessory for Outset Medical’s Tablo Hemodialysis System.
The Tablo Hemodialysis System is for use in patients with acute and/or chronic renal failure in an acute or chronic care facility. It requires only access to tap water and a standard electrical outlet, allowing dialysis to be delivered anytime, anywhere.
The TabloCart accessory is available in two versions, one with water prefiltration capabilities and another with added storage. It can adapt to varying water conditions and features enhanced maneuverability, including 360-degree rotating wheels.
“With the FDA’s clearance of TabloCart with prefiltration, we have delivered features to the Tablo Hemodialysis System that increase the flexibility for nurses and health systems to serve patients from the [intensive care unit] to the bedside,” said Leslie Trigg, chair and chief executive officer of Outset Medical.






 © 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.
© 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.