
The U.S. Food and Drug Administration approved dabrafenib in combination with trametinib for anaplastic thyroid cancer that cannot be removed by surgery or has metastasized and is BRAF V600E mutation-positive.
Both agents are approved alone or in combination to treat BRAF V600 mutation-positive metastatic melanoma, and they are approved for use in combination to treat BRAF V600E mutation-positive metastatic non-small cell lung cancer.
The recent approval was based on results from 23 evaluable patients; 57% experienced a partial response to this combination and 4% experienced a complete response. Among nine of the 14 patients who responded to this combination (64%), there were no significant tumor growths for six months or longer.
The most common adverse events associated with these agents are pyrexia, rash, chills, headache, arthralgia, cough, fatigue, nausea, vomiting, diarrhea, myalgia, dry skin, decreased appetite, edema, hemorrhage, hypertension, and dyspnea.
Source: FDA


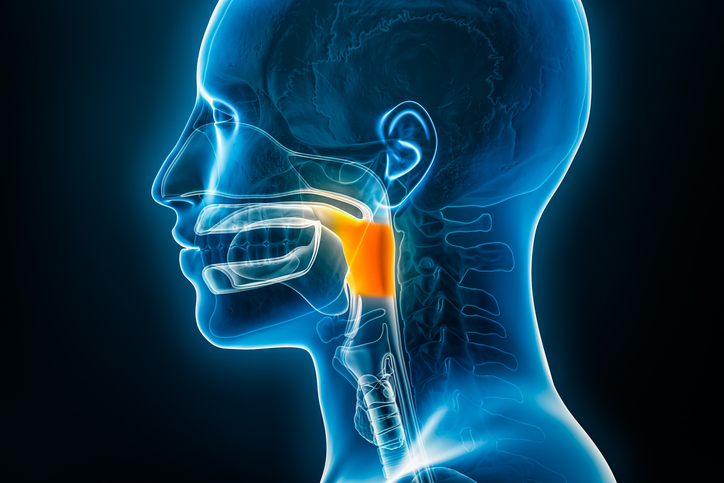



 © 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.
© 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.