Researchers from Osaka University in Japan have recently identified a gene that could play a pivotal role in developing targeted therapies for hepatoblastoma. This rare form of liver cancer affects only a few patients per million but is the leading cause of liver cancer amongst young children and infants, with a bulk of diagnoses being made before the patient is three years old. Aggressive cases of hepatoblastoma have few treatment options and yield low long-term survival rates, despite improvements in modern surgery and chemotherapy. With this new discovery, however, these Osaka researchers have taken an important step towards creating a treatment that targets hepatoblastoma. Their work was published on August 28 in the journal Nature Communications.
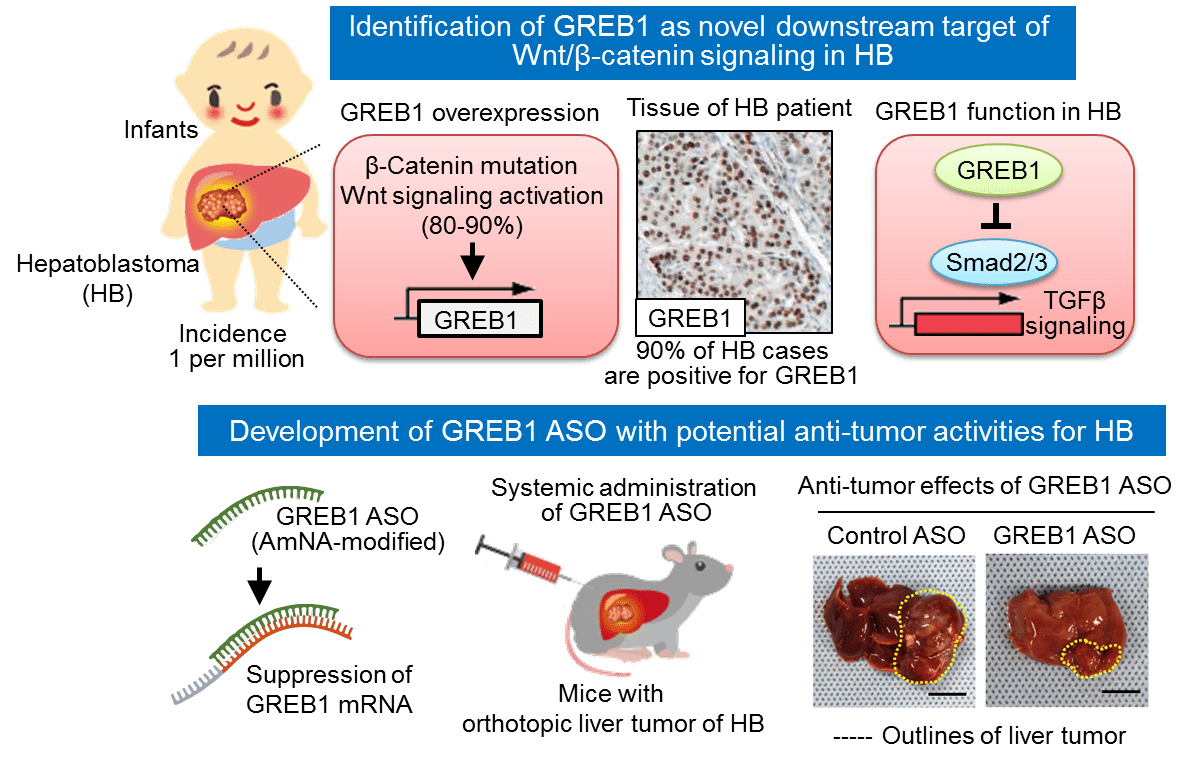
For the past 20 years, researchers have recognized that almost 90% of hepatoblastoma patients carry mutations in their β-catenin genes. Within the Wnt/β-catenin signaling pathway, β-catenin activates genes that are required for cellular proliferation. If regulatory mechanisms fail, β-catenin can build up, cause excessive cell growth, and lead to tumor formation. Mutations in genes involved with the Wnt/β-catenin pathway can lead to this accumulation of the protein and are common in many cases of cancer.
“We decided to screen uncharacterized Wnt/β-catenin target genes in liver tumor cells to try and identify novel genes with a role in the development of hepatoblastoma,” explained Shinji Matsumoto, lead author of the study. “One of the most abundantly expressed genes was growth regulation by estrogen in breast cancer 1 (GREB1), which is a well-known estrogen-responsive gene implicated in the growth of breast cancer cells.”
This gene has been thoroughly researched in relation to breast cancer but had not been identified as a target of the Wnt/β-catenin pathway prior to this work. In addition, researchers had not uncovered GREB1’s role in non-hormone-sensitive tumor development in the past either. After studying GREB1 in animal models, however, Matsumoto and colleagues were able to identify it as a key influencer in hepatoblastoma development.
“Overexpression of β-catenin in a mouse liver cancer model resulted in tumor formation and an increase in GREB1 expression,” said corresponding author Akira Kikuchi. “If we then suppressed the production of GREB1, we saw a decrease in hepatoblastoma cell proliferation and therefore fewer tumors in the study animals.”
Being that GREB1 is active in the cell’s nucleus, the Osaka team aimed to prevent tumor formation via amido-bridged nucleic acid-modified antisense oligonucleotides, which are small single-stranded DNA molecules that bind to mRNA to obstruct the production of target proteins. By targeting GREB1, these oligonucleotides were able to successfully decrease the production of GREB1 in the researchers’ experiment. As a result, the formation of hepatoblastoma tumors was suppressed.
Matsumoto, Kikuchi, and colleagues are optimistic that their work will lead to the development of a GREB1-specific treatment that can treat hepatoblastoma.
“We propose that GREB1 is a target molecule of Wnt/β-catenin signaling and required for hepatoblastoma progression,” the authors conclude.
[Release] Breast cancer gene a potential target for childhood liver cancer treatment https://t.co/hLKX9TaAod
— Osaka University (@osaka_univ_e) August 30, 2019




 © 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.
© 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.