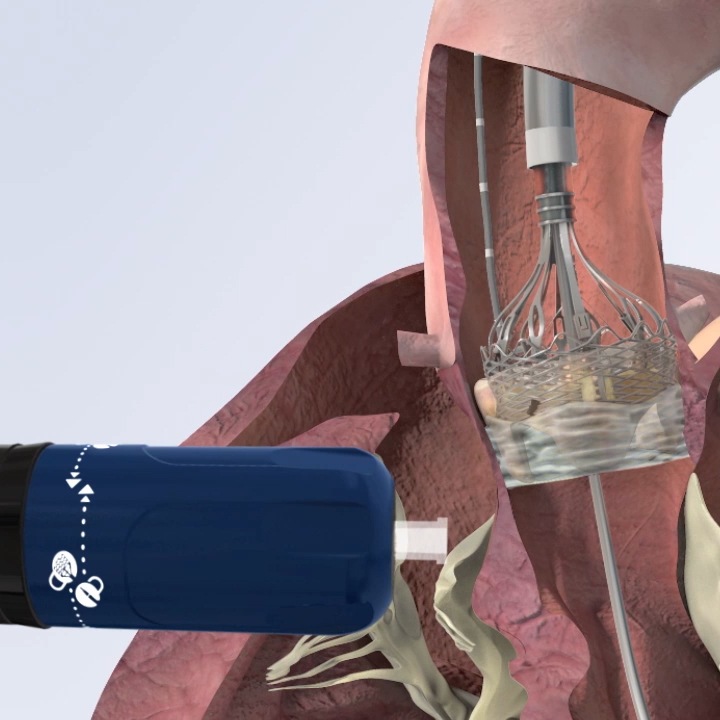
The FDA has approved a recapturable transcatheter valve for use in transcatheter aortic valve replacement (TAVR), according to a press release.
Boston Scientific announced that it has received approval for its Lotus Edge transcatheter valve from the Food and Drug Administration (FDA) for use in patients with severe aortic stenosis at high risk for surgical valve replacement, adding it to the Sapien and CoreValve families of transcatheter valves. A fourth competitor valve, the JenaValve, is currently under study in a pivotal trial. The Lotus Edge valve is repositionable, distinguishing it from its competitor products.
The Lotus Edge valve, according to the release materials, features a braided frame and adapted frame designed to conform to the patient’s aortic valve, thereby minimizing paravalvular regurgitation or leaking (PVL). The device can be recaptured by the operator once fully deployed. The company plans to launch the product in the United States in coming weeks, after a controlled launch in the European Union.
“We are thrilled to offer physicians in the U.S. and Europe the clinical benefits of the LOTUS Edge valve system for the treatment of their high-risk patients with severe aortic stenosis,” said Professor Ian Meredith, MD, executive vice president and global chief medical officer at Boston Scientific, said in a company press release. “This system provides physicians a high level of control over the delivery and deployment of the device and offers surgical-like PVL results to help ensure the best patient outcomes.”
Image courtesy of Boston Scientific.





 © 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.
© 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.